Intro to Water
The Importance of Water
Water is the main ingredient in coffee. Using good, clean water is important for making good coffee. When considering which water to use, there are two main considerations:
- Safety - this refers to boiler safety (scale buildup and corrosion), mainly for espresso.
- Taste - the composition of minerals in water can affect the taste of the final drink and may vary by preparation.
The Basics - Water Safety
When we refer to water safety, the focus is not on safety for consumption, though obviously you should always make sure that the water you are using is safe to drink. For coffee making, and particularly for espresso machines, safety refers to the hardness of the water that can cause scale buildup inside espresso machines and boilers as well as the corrosivity of the water that breaks down the boiler metal over time. Hard water can also cause scale buildup in kettles or automatic brewers.
Water general hardness (GH) is mostly a measure of the concentration of calcium and magnesium ions in the water. As water is heated and cooled, these minerals will deposit scale on any surface the water comes into contact with (pipes, boilers, kettles) and can eventually clog or damage these if not properly treated. Water hardness is typically measured in ppm (parts per million) as CaCO3 equivalent. That is the milligrams of equivalent CaCO3 per million milligrams (or per liter) of water solution. CaCO3 is just a standard mineral we convert to so that we can compare the hardness provided by one mineral to others with one unit of measurement.
As a general guideline, aim for ~50ppm hardness in your water, with >100ppm considered hard and not recommended for use in espresso machines
While the typical concern is water being too hard, water with too few minerals (very low ppm or distiller water) is not safe to use either, as it may corrode metals, with different boiler materials corroding at different rates.
How do I measure my water hardness?
In some areas, municipal water supplies post regular water reports which can be found by googling “town name water report”. The key factors to look for are general hardness (GH) and alkalinity (or KH). In the United States, this is often reported as parts per million (aka mg/L of CaCO3 equivalent). Keep in mind that these reports are not always accurate or up to date, but can be a good starting point - if your municipality reports high levels of hardness you should consider filtering or making your own water (more on water recipes below).
You can also measure your water using two fairly inexpensive methods:
- An electronic TDS (total dissolved solids) meter
- A water test kit that includes hardness strips
TDS meters measure the conductivity of your water and report the total ppm (not as CaCO3) in the water. This measures all dissolved solids in the water, not just magnesium and calcium, but can give a general direction of hardness - if your TDS ppm is between 50 and 100 you’re likely ok
Water test kits are available from espresso machine makers such as La Marzocco and will have test strips specifically for hardness minerals. The tests themselves will not be as precise as TDS measurement but can give a general sense of hardness minerals in your water
What do I do if my water is hard?
There are several options when considering water treatment / replacement:
- Water filters
- Store-bought mineral water
- Coffee water mineral packets
- Custom water recipes
Water filters can help reduce water hardness as well as improve overall water quality for flavor. There is a wide range of water filters, from pitchers such as ZeroWater all the way to plumbed in filtration systems. Keep in mind that if you use a total filtration (purification) system, like ZeroWater, reverse osmosis (RO) or de-ionization (DI), you should add minerals to your water post-filtration, as fully distilled water will both taste bad and may be harmful for your espresso machine (corrosive).
Some machine manufacturers recommend specific mineral water brands. Generally speaking it is hard to know the exact composition of minerals for different brands, but if your water is particularly hard, this may be a safer option.
Mineral packets / droppers are now widely available to remineralize RO water or distilled water. Companies such as TWW and Lotus offer products with specific mineral content for different brewing techniques. However, it should be noted that most pre-made mineral concentrates (like Lotus) use chlorides which can be very corrosive and so are not usually recomended for espresso machines.
Lastly, many aficionados like to make their own water, starting with RO or distilled water as a base and adding a specific mixture of minerals for their taste and brewing methods.
Continue reading below for a more advanced section on how to make your own water and current popular recipes.
Advanced - Making Your Own Water
The Simple Water Recipe
A longtime Espresso enthusiast, Dr. Pavlis, simplified the recipe as follows: 50-100mg/L of potassium or sodium bicarbonate. This water is scale free when used in an espresso machine prevents the need for regular descaling. A simple way to utilize this ratio is as follows:
Ingredients
4L Distilled or Deionized water
0.35g Potassium Bicarbonate
Method
- Put the potassium bicarbonate into the water
- Agitate
- Let rest for 5-10 mins (This ensures sufficient time for the potassium bicarbonate to properly dissolve into the water)
That’s it. Really.
The Why
The premise here is that the water acts simply as a carrier for the compounds you are trying to extract from your beans. We start with distilled or de-ionized water as a base because these have little to no TDS (a measure of the amount of dissolved particles of other substances are in the water). In order to neutralize the corrosive effect this will have on your machine, and to improve the extraction of desirable coffee compounds, Dr. Pavlis settled on the use of potassium (or sodium) bicarbonate. In this application the bicarbonate acts as a “buffer”, bringing the pH of the distilled water closer to 7 (neutral pH) while also raising TDS a little bit.
The “But my scale won’t measure that accurately”
A shortcoming of the recipe provided above is the difficulty of consistently measuring 0.35g of the buffer. So, yet more perfection and simplification seeking enthusiasts reached the conclusion that a concentrate of buffer can be used to simplify the measurements needed.
To make a 10,000x ppm concentrate, mix 20g of potassium bicarbonate into 1L of distilled water.
To use the concentrate, start with 5mL of concentrate and add water to a total volume of 1L. To make 4L of water, start with 20mL of concentrate and add water to a total volume of 4L.
The More
Some espresso enthusiasts have found the “Pavlis Water” recipe is lacking a component. The thought here is that calcium or magnesium (commonly considered “hardness” minerals), in carefully controlled doses, change the taste of the brewed coffee. I will stop short of making any qualitative judgement between this approach and the one outlined above. Since there are myriad ways to introduce hardness chemicals into your water, we’ll continue to add water resources here on the wiki.
Water 2: A Practical Guide of Understanding Water for Dialing in Coffee
by azeotropes, chill’d, and sagebush
TLDR:
If RPavlis water mentioned in the beginning water entry has a somewhat weird taste, try these other recipes. My current favorite for espresso is the TWW Espresso Inspired.
Epsom Salt = MgSO4·7H2O
Baking Soda = NaHCO3
Potassium Bicarbonate = KHCO3
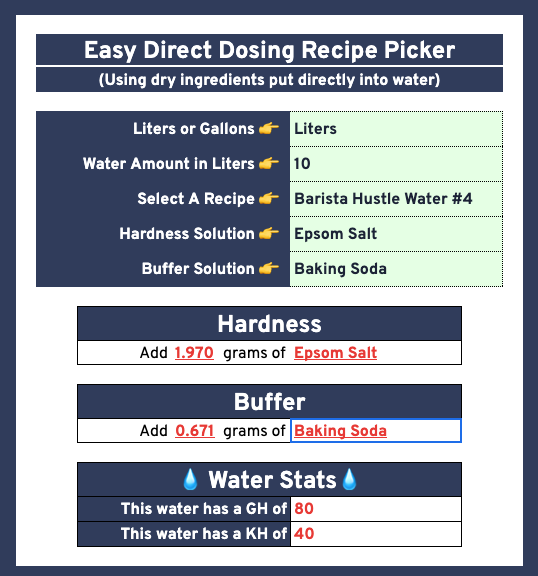
Direct dosing
The method I am proposing is to direct dose into the final container without any concentrates. You add these amounts, and then fill the water to the proper volume. Scale up or down as needed. 10L is used for easy measurements using 0.1g accurate scales. For smaller measurement, jewelry scale that goes to 0.001g could be used. AWasteOfCoffee recommended these, and I got them. Concentrates could also be used if your scale does not weigh fine enough. If only a 0.1g scale is on hand, then I recommend starting with RPavlis as a forgiving water recipe. (0.38g Potassium Bicarbonate to 1 gallon of water). Aim for 0.4 to 0.5g.
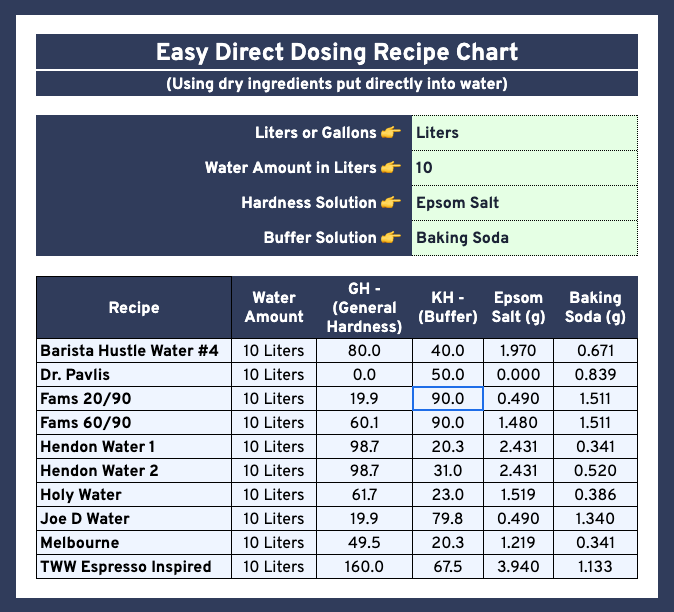
| Name | Volume | Epsom Salt (g) | Baking Soda (g) | Potassium Bicarbonate (g) | GH (mg/L of CaCO3 equiv.) | KH (mg/L of CaCO3 equiv.) | Comments |
|---|---|---|---|---|---|---|---|
| “Holy” Water | 10 Liter | 1.520 | 0.000 | 0.460 | 62 | 23 | Targeted towards Filter |
| ” | 1 Gallon | 0.575 | 0.000 | 0.174 | 62 | 23 | ” |
| Melbourne Water | 10 Liter | 1.220 | 0.340 | 0.000 | 49.5 | 20.2 | General Purpose Water for Both |
| ” | 1 Gallon | 0.461 | 0.129 | 0.000 | 49.5 | 20.2 | ” |
| Hendon Water | 10 Liter | 2.430 | 0.520 | 0.000 | 99 | 31 | Targeted towards Espresso |
| ” | 1 Gallon | 0.922 | 0.197 | 0.000 | 99 | 31 | ” |
| Barista Hustle Water #4 | 10 Liter | 1.970 | 0.671 | 0.000 | 80 | 40 | General Purpose Water for Both |
| ” | 1 Gallon | 0.745 | 0.254 | 0.000 | 80 | 40 | ” |
| TWW Espresso Inspired | 10 Liter | 3.940 | 0.000 | 1.350 | 160 | 67.5 | Targeted towards Espresso |
| ” | 1 Gallon | 1.489 | 0.000 | 0.510 | 160 | 67.5 | ” |
| RPavlis | 10 Liter | 0.000 | 0.000 | 1.000 | 0 | 50 | Targeted towards Espresso |
| ” | 1 Gallon | 0.000 | 0.000 | 0.378 | 0 | 50 | ” |
| Fam’s 29th Wave | 10 Liter | 0.493 | 1.511 | 0.000 | 20 | 90 | Targeted towards Light Roast Espresso |
| ” | 1 Gallon | 0.186 | 0.571 | 0.000 | 20 | 90 | ” |
| Fam’s 69th Wave | 10 Liter | 1.478 | 1.511 | 0.000 | 60 | 90 | Targeted towards Light Roast Espresso |
| ” | 1 Gallon | 0.559 | 0.571 | 0.000 | 60 | 90 | ” |
Use Spreadsheet Calculators here to look up the above or craft your own waters!
Read Detailed Explanations here
- Fam’s recipe above has been modified for easier use from potassium bicarbonate to baking soda. According to Sagebush “Baking Soda smoothes out the brightness quiet a bit. Potassium is more flavour transparent but adds a distinct metallic taste.” Use the factors below to switch back to using potassium bicarbonate if desired.
Conversion of Baking Soda vs Potassium Bicarbonate
The use of baking soda and Potassium bicarbonate for this purpose is for alkalinity. To switch from one value to another you simply multiply it by a factor. The molecular weight of baking soda is 84 g/mol. The molecular weight of potassium bicarbonate is 100 g/mol.
Baking Soda -> Potassium Bicarbonate = (weight you wish to convert) * (mw of Potassium Bicarbonate / mw of Baking Soda) = (weight) * 100/84
Potassium Bicarbonate -> Baking Soda = (weight you wish to convert) * (mw of Baking Soda / mw of Potassium Bicarbonate) = (weight) * 84/100
Example #1 Converting Barista Hustle #4 1 Gallon from Baking Soda to Potassium Bicarbonate
Weight required with Baking Soda: 0.254 g.
0.254 g * 100/84 = 0.302 g Potassium Bicarbonate
Example #2 Converting RPavlis Water 1 Gallon from Potassium Bicarbonate to Baking Soda
Weight required with Potassium Bicarbonate: 0.378 g.
0.378 g * 84/100 = 0.317 g Baking Soda
Using Concentrates
An alternative to direct dosing is to pre-mix concentrates, and use those for your recipes. Using 1,000 or 10,000 ppm concentrates makes trying new water recipes easy.
For example, to make RPavlis 0/50 (GH/KH) water, take 5g of 10,000x KH concentrate and add water up to a total volume of 1L. Holy water (62/23) can be made by adding 6.2g of 10,000x GH concentrate to 2.3g of 10,000x KH concentrate and adding water to a total volume of 1L.
If scale precision is an issue, 1,000ppm concentrates can be made by diluting 100ml of 10,000x concentration with water to a total volume of 1L.
GH (aka General Hardness) Concentrates:
| Compound | Mass (g) | Water volume (mL) | Concentration (mg/L of CaCO3 equiv.) |
|---|---|---|---|
| Magnesium Sulphate Heptahydrate (Epsom Salt) | 24.63 | 1,000 | 10,000 |
| Magnesium Chloride Dihydrate | 13.11 | 1,000 | 10,000 |
| Magnesium Chloride Hexahydrate | 20.31 | 1,000 | 10,000 |
| Calcium Chloride Dihydrate | 14.69 | 1,000 | 10,000 |
| Calcium Sulphate Dihydrate (Gypsum)* | 17.20 | 1,000 | 10,000 |
| Calcium Hydroxide | 7.40 | 1,000 | 10,000 |
* Note: Only around 3.6g of gypsum will fully dissolve in 1,000mL of water at room temperature. This means the highest concentration, fully dissolved gypsum concenrate you can make is around 2,092mg/L of CaCO3 equiv. 1.72g of gypsum in 1,000mL of water would make a safely dissolved solution with concentration of about 1000mg/L of CaCO3 equiv.
KH (aka Carbonate Hardness aka Buffering capacity) Concentrates:
| Compound | Mass (g) | Dilute volume (mL) | Concentration (mg/L of CaCO3 equiv.) |
|---|---|---|---|
| Sodium Bicarbonate | 16.79 | 1,000 | 10,000 |
| Potassium Bicarbonate | 20.00 | 1,000 | 10,000 |
Key Point:
Adjust hardness and alkalinity of coffee to match your tastes. It can be used as one of the final steps to dial in a certain taste for a specific machine and grinder combination. The most optimal water could change with coffee ratios. This page is a work in progress to try and gather some heuristics to build intuition on how water for coffee will work.
Increasing general hardness will increase intensity of flavors extracted. Increasing alkalinity will reduce harsh acidity associated with sourness.
The Idea
Coffee is primarily composed of water. Even having good beans and a good grinder, if poor water is used, it will result in a bad coffee. Water quality and minerals varies depending on the area that the user is living in. Some areas just naturally have the ideal soft water for coffee like San Francisco or Seattle. Others like Los Angeles will have water that will make coffee taste bland. One way to get around this is to craft your own water mixture. This page will guide through the idea of remineralizing Reverse Osmosis (RO) or Distilled Water.
The main components we will be looking at are:
Distilled or De-ionized water
Magnesium Sulfate (Epsom Salt)
Potassium Bicarbonate
Sodium Bicarbonate (Baking Soda)
Chlorides and calcium are also used in the remineralization of water to achieve different taste profiles. They are absent from this page because this would give undesirable effects for espresso machines. There may be advanced ways of adding chloride and calcium such that they won’t scale, but that requires some additional math and equations that are out of scope of this document.
Hardness: The concentration of divalent cations (positive ions) - mostly magnesium and calcium - will give the total (or general) hardness. More hardness will generally make the coffee’s flavor more intense.
Alkalinity: This will add more buffer. A higher alkalinity number will help reduce acidity.
There are online calculators and spreadsheet to calculate alkalinity and hardness. They are linearly independent. But for thoroughness, look at the end for an example to make 50 KH (alkalinity) and 100 GH (hardness).
Practical Example:
Dialing in Kenya Nyeri AB Giakanja
Rao Allonge Parameters:
- Roast level: light
- 18:90, 1:5 ratio
- 92°C
- 6-9 bars of pressure.
- 4.5 ml/s flow rate
Attempt #1
Water #1: RPavlis Water
0 GH 50 KH
The shots made with RPavlis water felt very muted in acidity. It made me wonder if adding some hardness would help bring some more of the bean compounds out. It tasted a certain kind of emptiness. I decided to follow another common recipe that I knew for sure would not scale.
Attempt #2
Water #2: Barista Hustle #4
80 GH 40 KH
The addition of Mg to hardness caused a ton of acidity. It felt very sour. It tasted a lot like lemon juice. There were some good flavors, but I really felt the need to mute the acidity.
Attempt #3
Keeping water constant
80 GH 40 KH
Temperature raised to 97°C
I was thinking that increasing the temperature higher could extract more compounds and may balance out the acidity. This did not work out well. Currently, at this point, I was thinking of heading towards RPavlis water, while reducing the hardness.
Attempt #4
Water #3: Custom Water
40 GH 50 KH
Back to 92°C.
The alkalinity was raised from 40 to 50. Hardness was halved from 80 to 40. The shot itself was still more acidic than I would like, but it was muted more than the previous water recipe. I am not sure if I can contribute this towards reducing the hardness or increasing the alkalinity. This is because I changed two variables at a time. But it was moving in the right direction. Another coffee aficionado mentioned full TWW Espresso Profile helped tune down this acidity. That is where I headed to next.
Attempt #5
Water #4: TWW Espresso Blend Inspired Water
160 GH 67.5 KH
This is great. The acidity from the 2nd attempt shot with Barista Hustle #4 water are gone. It is a bit sour/acidic in the beginning, followed by a slight bitter after taste. I am currently stopping here for now in my dialing in process, but I may explore softer waters later.
Other Water Examples
Johnathan Gagné of Coffee ad Astra has this graph for certain water hardness and alkalinity levels. You can use a spreadsheet and/or calculator to determine how to create your own water mix.
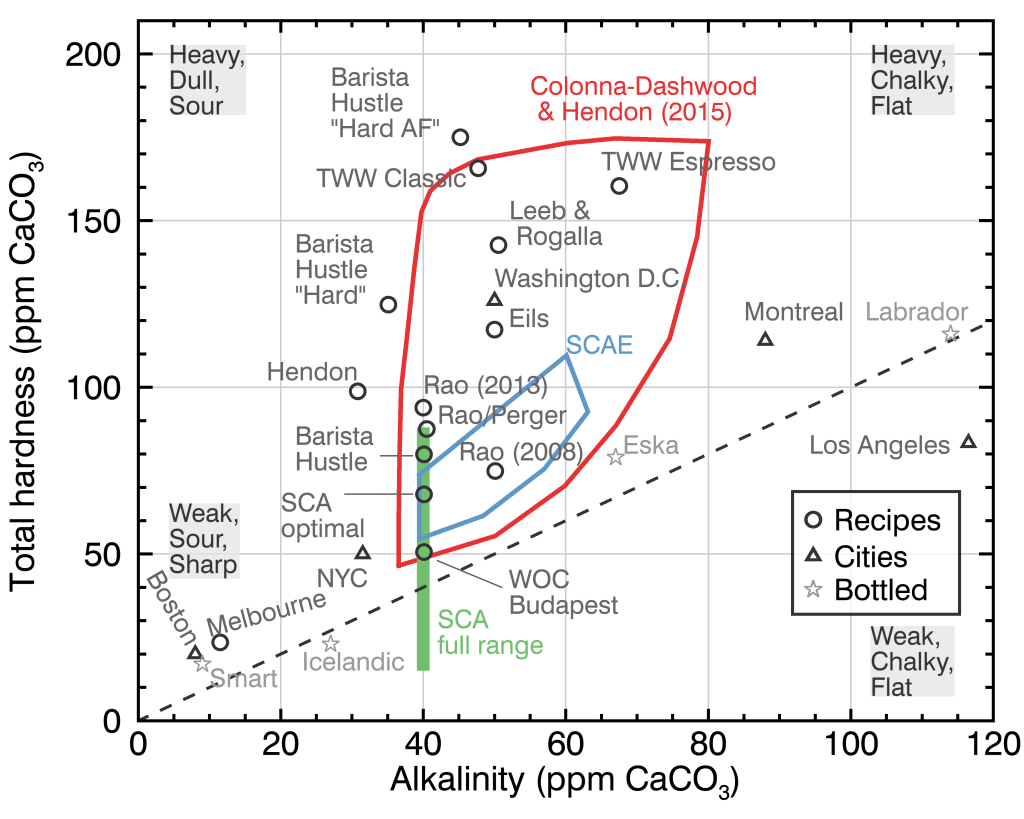 Graph from Coffee ad Astra’s Water Article
Graph from Coffee ad Astra’s Water Article
Tasting Minerals by Sagebush
Please note that Sagebush is using a manual machine (Cafelat Robot). This is much less prone to scaling and corrosion compared to other espresso machines. What does scale can be easily cleaned off.
Hardness and Buffer Design of Experiments
I compared different extreme amounts of hardness and buffer, using MgSO4 and NaHCO3. I did nine samples, 0-100-200 hardness (GH) and 0-50-100 buffer (KH).
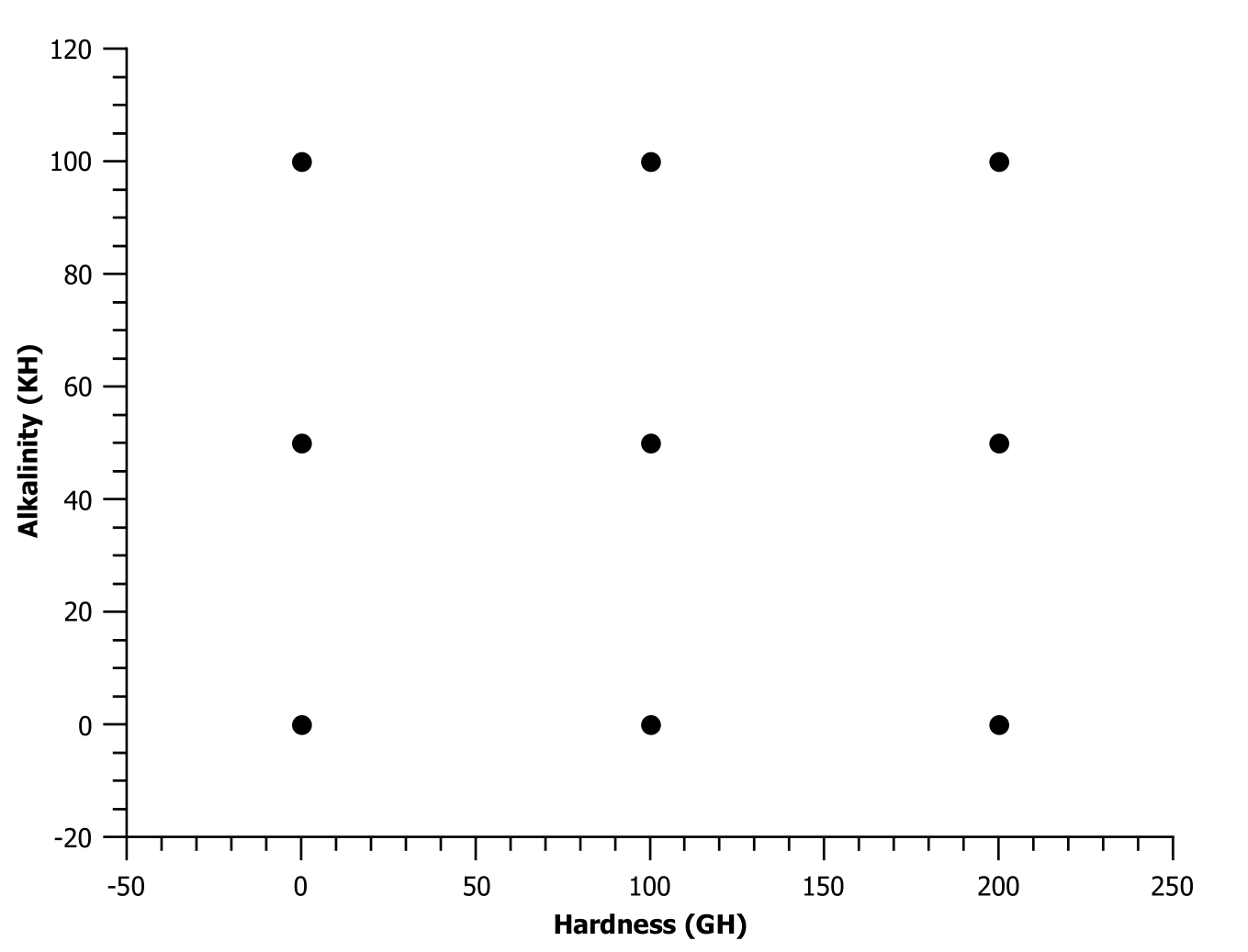
- 0GH/0KH was pretty light and acid forward. I would not call it hollow because there was a whole spectrum of flavors, but all nuances were very light with lots of ‘space’.
- 200GH/0KH was very intense and full but also acidic. Very exciting and attention grabbing, kind of wild.
- 0GH/100KH was light, mild, mellow, comforting.
- 200GH/100KH was intense but kind of ‘cloudy’, a very dense and strong wall of flavors, a bit like a crazy natural.
I did like 0GH/100KH, 100GH/50KH, 200GH/0KH and 200GH/50KH (for different purposes of course), the other options felt too unbalanced. 0GH/0KH and 200GH/100KH, while being on the ‘balanced ratio’ axis, did not produce balanced or enjoyable results (to me). The other extremes, 0GH/100KH and 200GH/0KH, however where enjoyable for their specific effects.
My takeaways are:
- ~80GH/40KH is a good middle ground
- A wide range of TDS can produce good results
- Hardness and buffer should be altered inversely to support the effects of each other.
Practical implications are: highly acidic beans, or heavy naturals, will probably benefit from going low GH/ high KH, while balanced coffees can be amped up with high GH/ low KH. A mellow morning coffee can benefit from the former, while attentive pleasure drinking can benefit from the latter.
My experience is that buffer managed the very top acidity, the biting sourness, while the hardness intensifies everything else, the ‘meat and bones’. Too low hardness makes it watery, while too high hardness amplifies and compresses the flavors. Too low buffer had biting sourness while too much buffer is just boring and uninteresting.
Tasting Minerals
I compared MgSO4, MgCl2, CaSO4 and CaCl2 using 80 mg as CaCO3 hardness and 40 buffer (NaHCO3). The differences are very nuanced. I’d say Mg tastes more exciting/harsh than Ca which is more mellow/muted. Cl tastes more creamy than SO which is more neutral. I had a hard time finding the difference between MgCl2 and CaSO4, it is very nuanced.
I did a comparison of different buffer minerals, namely NaHCO3, KHCO3, Ca3C12H10O14 (calcium citrate) and Na3C12H10O14 (sodium citrate). The first observation is that calcium citrate barely dissolves, the concentrate needs to be shaken up well before usage. For tasting I did a cupping with a 1:14 ratio and 74/40 water, using MgCl2 as a hardness source.
Taste wise NaHCO3 was the smoothest. KHCO3 seemed more ‘neutral’, it did not alter the coffee taste, but it added a slight bitter metallic taste. Calcium citrate was by far the worst, rather bitter, do not recommend. Sodium citrate was very good, brighter than the rest with a slight added citrus flavour. I’d recommend NaHCO3 for more smoothness and sodium citrate for more brightness
The tasting was done blind, but it confirmed all taste attributes that I have found in the literature so far (except for calcium citrate which I have not found in the literature, I just reasoned that it could probably be used as a buffer source)
Explanations of Waters in the Water Recipe Table
These three were submitted by Strivefortone (Holy Water, Melbourne Water, and Hendon Water)
- Melbourne water - soft - good for espresso, works well with filter
- Holy water - soft - works amazing for filter, ok with espresso
- Hendon water - harder - works amazing for espresso, a little too hard maybe for filter
Holy Water
Holy water was my best attempt at a universal kind of water - the project I developed it for was going to have frozen coffees for both filter and espresso, with espresso made on a GS3 with its own reservoir. Only Holy water was amazing for filter and we never got the roast dialled right for espresso.
If aiming for filter, try this water out. It works very well.
Melbourne Water
Melbourne Water worked really well for almost all the roast styles at Ali (a cafe shop), but definitely suffered when I used other roasters coffee.
Hendon Water
Revised Hendon water is working very well for turbos. The original formula still stands up to most espresso styles, but extracts way too much for filter.
Barista Hustle Water #4
One of the water that started it all from Barista Hustle. This water works as a nice general purpose water.
TWW Espresso Inspired
A copy of the hardness and alkalinity of the TWW Espresso Blend using epsom salt and a buffer. It is a cost effective way to copy the TWW espresso profile, while not being an exact copy.
RPavlis
A recipe meant to help keep the machine from scaling, while also providing enough mineral content to not harm the espresso machine. The original recipe uses potassium bicarbonate because it is believed that sodium bicarbonate (baking soda) will impart some flavors. It surprisingly works very well for pulling espresso. It tastes a bit weird with longer ratio coffee (like filter). It is perhaps the most forgiving recipe to create, and would work anywhere from 50 KH to 100 KH. If you only have a 0.1g scale, probably use this water first.
Fam’s 29th and 69th Wave
Based on “holy water” (60 ppm as CaCO3 hardness, 20 ppm as CaCO3 buffer, short 60/20), which Fam found to be really good for filter, he arrived at 60/90 for espresso. The amount of buffer was scaled up from holy water because of the volume of an espresso shot is much less than that of filter. Hardness is postulated to be the “extraction power” of the water and is kept the same for both.
This can be a bit harsh for high clarity burrs, this can be fixed by reducing the hardness, some people like 20/90 a lot, which is more gentle.
For ease of use and taste, epsom salt and baking soda are used.
60 hardness was based off my preference for Strivefortone’s Holy water for filter coffee; my burrset extracts ~22-24% for its sweetspot and thats about the maximum hardness I got before running into harsh flavors. Lots of juicyness.
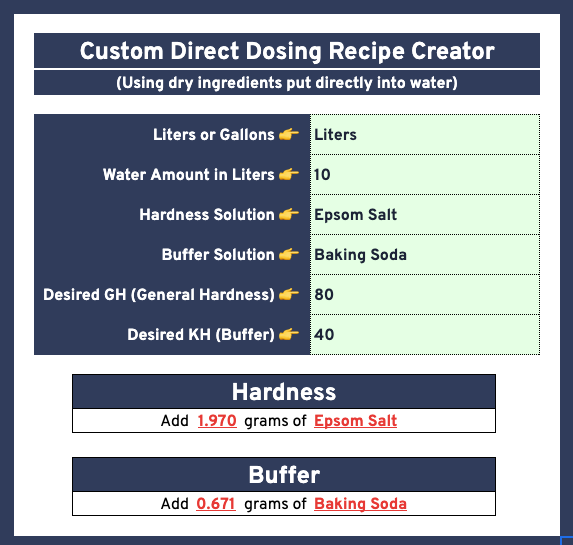
Making Yor Own Custom Waters
EDIT(Nov 18, 2021), Chris has created an updated spreadsheet that combined the single dosing sheets into a nice form. It can be found at https://coffeewater.app/. The same idea still applies below.
Since last updated (~July 2021, now ~Sep 2021), I’ve moved from the TWW Espresso Inspired to 75/25 GH/KH and now back to Barista Hustle #4 (80/40). But say you want to try recipes like 20/90, 60/90 or something that is not listed in the tables. There are ways to calculate it manually, but the easiest I have found for myself is to use the spreadsheets already written. We do not need to reinvent the wheel. That said, some people find it confusing to not know what the spreadsheets are doing under the hood. Still others simply prefer to do it themselves. If that is you, read the Custom Water Math section. Jonathan Gagné and David Seng have made their spreadsheet calculators. They do work either in Google Docs or Excel. I have found David Seng’s calculator to be very advanced for my needs, so I will document using Gagné’s.
The first step is to decide if you want to use sodium bicarbonate (baking soda) or potassium bicarbonate as your buffer. You can try both to see if there are any flavor differences. Then go to the appropriate sheet in the spreadsheet. I will detail the steps for 1 gallon below (3780 mL), but feel free to change it to your end target, say 5L (5000 mL) or 10 L (10000 mL).
First make a copy of the Water for Coffee Crafter. Then go to either the “Epsom_Baking” (Sodium Bicarbonate) or Epsom_KHCO3” (Potassium Bicarbonate)
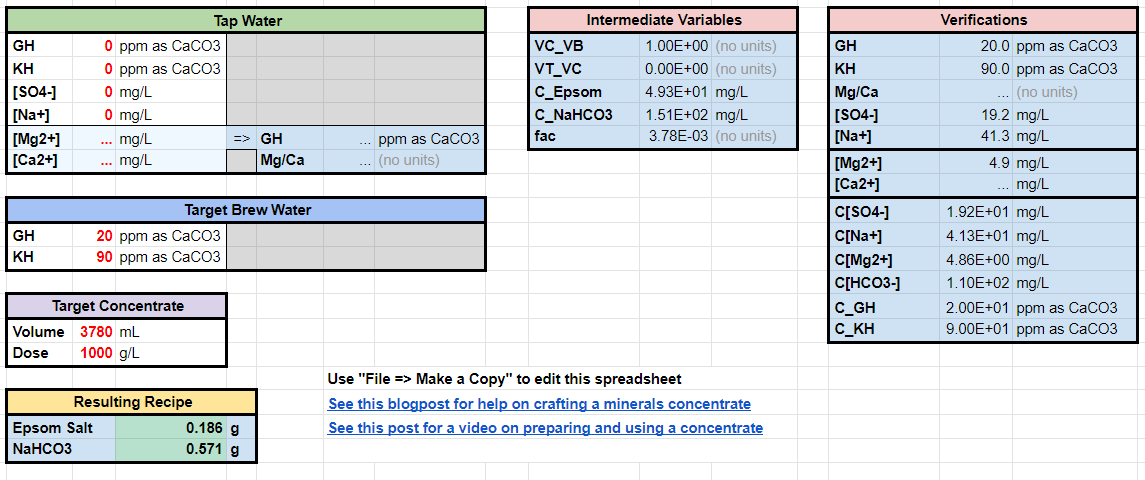
Here is an example of 20/90 with Epsom Salt and Baking Soda. You may check how close (or far) the values match the table of water recipes above.
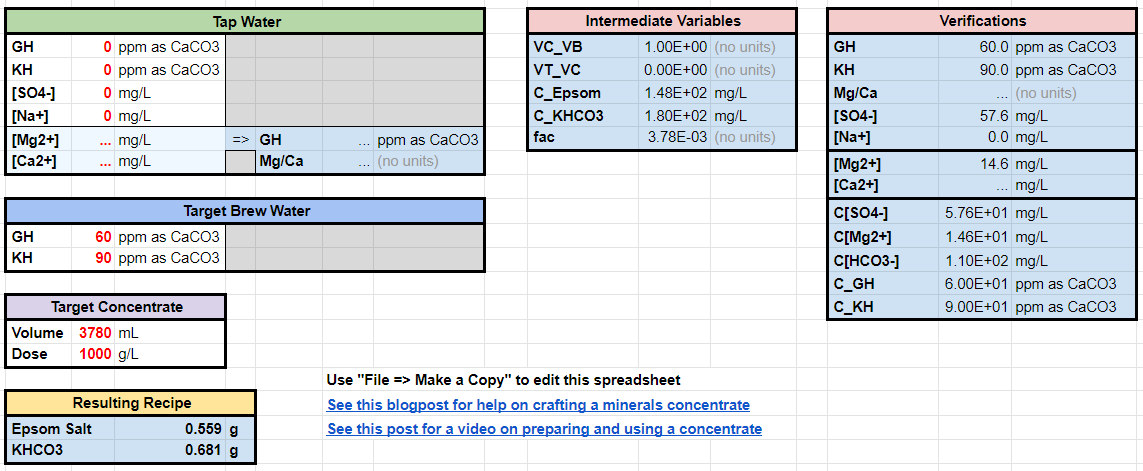
Here is an example of 60/90 with Epsom Salt and Potassium Bicarbonate.
Spreadsheet Calculators
Example screenshots below:
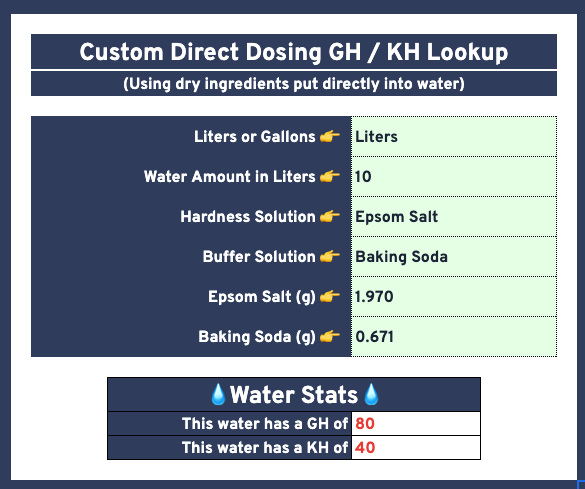
Custom Water Math
When making custom water recipes, for individual mineral concentrates, combined concentrates, or direct dosing, people often want the answer to one of the following questions:
- If I want c ppm of hardness from ion i, how much mass m of mineral n do I need and in how much water w should I dissolve it?
- If I put mass m of mineral n in an amount of water w, what ppm c will the solution be?
These two questions are the opposites of each other and as such, the same reversible process can be used to answer both. Note that in the following section, “salt” and “mineral” are synonyms.
- First we’ll cover what to do - the steps - and why each step is needed.
- Then we’ll show the equations you need to carry out each step.
- Then we’ll go through a practical example, applying the equations.
Steps & Reasoning
Target ppm as CaCO3 > salt mass (forwards)
- (equation 2) CaCO3 salt mass concentration > hardness ion mass concentration
In this step, we are figuring out what part of the total mass of CaCO3 comes just from the ion we care about.
If we want GH, we care about the Ca2+ ion
If we want KH, we care about the (CO3)2- ion - (equation 3) hardness ion mass concentration > hardness ion molarity
Here, we are converting the mass concentration of the relevant ion, to a number of ion molecules concentration (molarity)
(because we care about hardness and the hardness contribution is a function of charge - which is dependent on number of molecules, not mass…
i.e. a heavy ion could contribute the same hardness as a light one) - (equation 4) hardness ion molarity > hardness equivalents concentration (HE)
Here we are converting the molarity to hardness equivalents concentration (which is based on charge)
(because hardness contribution is what we actually care about,
i.e. we want some amount of salt that gives the equivalent hardness as some amount of CaCO3)
**Note: Here we start to do each step in reverse, but with the ion in our solute instead of the ion in CaCO3. **
- (equation 4 reverse) hardness equivalents concentration (HE) > salt ion molarity
Here we are converting from hardness (related to charge) to number of salt hardness ion molecules concentration (molarity)
(because we are adding ions via salt, not hardness directly) - (equation 3 reverse) salt ion molarity > salt ion mass concentration
Here we are converting molarity to mass concentration (because what we measure on the scale is mass, not molecules) - (equation 2 reverse) salt ion mass concentration > salt mass concentration
Here we are converting salt hardness ion mass concentration to total salt mass concentration (because we aren’t adding/measuring individual ions, we are adding/measuring salt) - (equation 1 reverse) salt mass concentration > salt mass
Here we are converting salt mass concentration to salt mass
(because we are making a specific amount of concentrate solution that requires a specific amount of salt)
Salt mass > result ppm as CaCO3 (backwards)
- (equation 1) salt mass > salt mass concentration
Here we convert from a specific amount of salt to a mass concentration
(because at the end we want the concentration of equivalent CaCO3) - (equation 2) salt mass concentration > salt ion mass concentration
Here we figure out how much of the total mass of the salt comes from the hardness ion we care about.
For example, in baking soda, the hardness ion is (HCO3)1- - (equation 3) salt ion mass concentration > salt ion molarity
Here we are converting the mass concentration of the relevant ion, to a number of ion molecules concentration (molarity)
(because we care about hardness and the hardness contribution is a function of charge - which is dependent on number of molecules, not mass…
i.e. a heavy ion could contribute the same hardness as a light one) - (equation 4) salt ion molarity > hardness equivalents concentration (HE)
Here we convert the molarity to hardness equivalents concentration (which is based on charge)
(because hardness contribution is what we actually care about,
i.e. we want to know how much CaCO3 gives the equivalent hardness as the amount of salt we started with)
**Note: Here we start to do each step in reverse, but with the ion in our solute instead of the ion in CaCO3. **
- (equation 4 reverse) hardness equivalents concentration (HE) > hardness ion molarity
Here we are converting from hardness (related to charge) to number of number of relevant ion molecules (from CaCO3) concentration (molarity)
(because the number we want is an amount of CaCO3, not charge) - (equation 3 reverse) hardness ion molarity > hardness ion mass concentration
Here we convert molarity to ion mass concentration (because ppm is in terms of mass, not molecules) - (equation 2 reverse) hardness ion mass concentration > CaCO3 salt mass concentration
Here we convert from ion mass concentration to CaCO3 mass concentration
(because ppm is measuring mass of CaCO3, not only the mass of only the relevant ion)
A concrete example of the “forward” process follows the equations below:
Equations
- Salt Mass > Salt Mass Concentration
- equation:
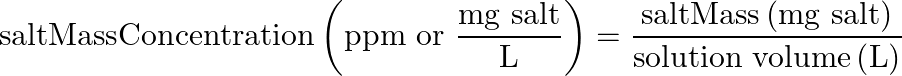
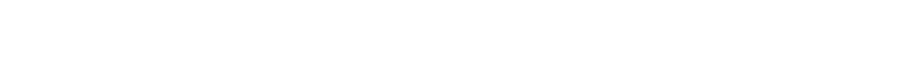
- reverse equation:


- equation:
- Salt Mass Concentration > Ion Mass Concentration
- main equation:
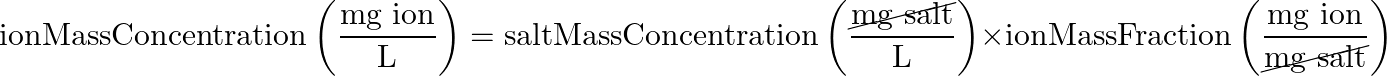

- reverse main equation:
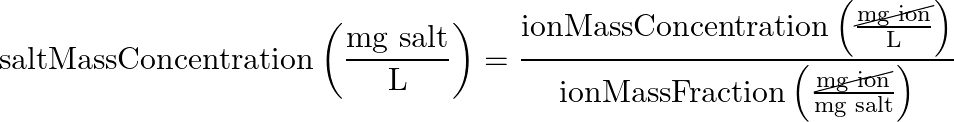
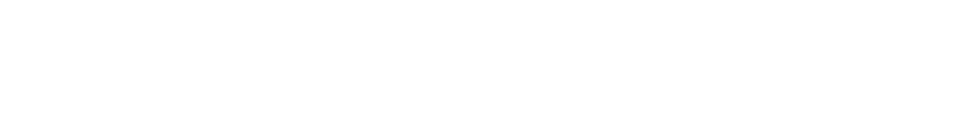
- Ion Mass Fraction:
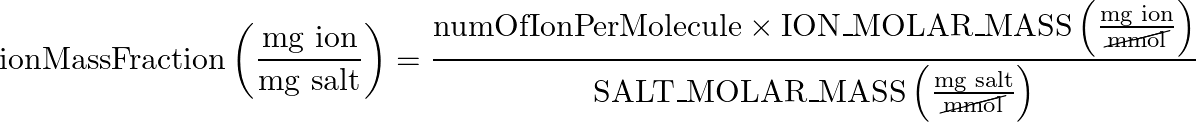

- main equation:
- Ion Mass Concentration > Ion Molarity
- equation:
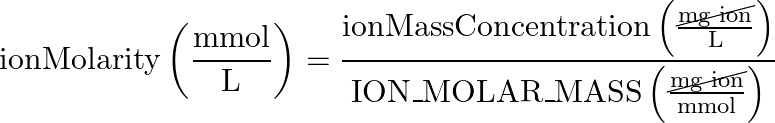
- reverse equation:
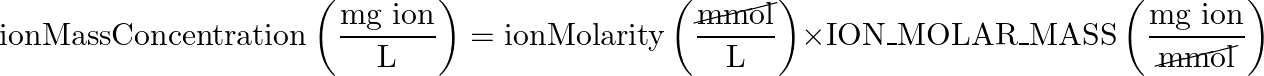

- equation:
- Ion Molarity > Hardness Equivalents Concentration (HE)
- equation:
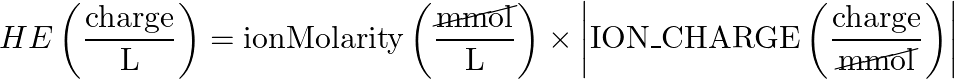

- reverse equation:
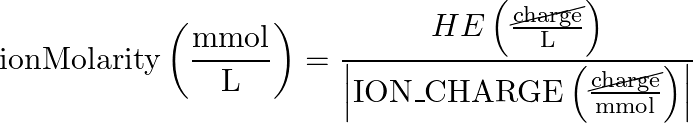
- equation:
Forward Example
Let’s say we want 20,000ppm as CaCO3 of KH from baking soda (NaHCO3). We want to know how much baking soda we need in how much water. So we are going forwards in the process (CaCO3 salt mass concentration »> NaHCO3 salt mass) (2, 3, 4, 4, 3, 2, 1)
-
CaCO3 salt mass concentration > CO3 ion mass concentration
In this step, we are figuring out what part of the total mass of CaCO3 comes just from the ion we care about.
We want KH, so the ion that gives us that is the (CO3)2- ion in CaCO3. so…
CO3MassConcentration (mg CO3/L) = 20,000 (ppm or mg CaCO3/L) * CO3MassFraction (mg CO3 / mg CaCO3)with a
CO3MassFraction (mg CO3 / mg CaCO3) = (1 * 60.0089 (mg CO3 / mmol)) / 100.0869 (mg CaCO3 / mmol)
= 0.5995679754 (mg CO3 / mg CaCO3)so
CO3MassConcentration (mg CO3/L) = 20,000 (mg CaCO3/L) * 0.5995679754 (mg CO3 / mg CaCO3)
= 11991.359508 (mg CO3/L) - CO3 ion mass concentration > CO3 ion molarity
Here, we are converting the mass concentration of the relevant ion, to a number of ion molecules concentration (molarity)
(because the hardness contribution is a function of charge - which is dependent on number of molecules, not mass…
i.e. a heavy ion could contribute the same hardness as a light one)
CO3Molarity (mmol/L) = CO3MassConcentration (mg CO3/L) / 60.0089 (mg CO3/mmol)
= 11991.359508 (mg CO3/L) / 60.0089 (mg CO3/mmol)
= 199.8263508913 (mmol/L) -
CO3 ion molarity > hardness equivalents concentration (HE)
Here we are converting the molarity to hardness equivalents concentration (which is based on charge)
(because hardness contribution is what we actually care about,
i.e. we want some amount of salt that gives the equivalent hardness as some amount of CaCO3)
HE(charge/L) = CO3Molarity (mmol/L) * |CO3_CHARGE (charge/mmol)|with
CO3_CHARGE (charge/mmol) = -2because the CO3 ion has a charge of negative 2 (-2)so
HE (charge/L) = 199.8263508913 (mmol/L) * |-2 (charge/mmol)|
= 199.8263508913 (mmol/L) * 2 (charge/mmol)
= 399.6527017826 (charge/L)
**Note: Here we start to do each step in reverse, but with the ion in our solute instead of the ion in CaCO3. **
-
(step 4 reverse): hardness equivalents concentration (HE) > HCO3 ion molarity
Here we are converting from hardness (related to charge) to number of salt hardness ion molecules concentration (molarity)
HCO3Molarity (mmol/L) = HE (charge/L) / |HCO3_CHARGE (charge/mmol)|with
HCO3_CHARGE (charge/mmol) = -1because the HCO3 ion has a charge of negative 1 (-1)so
HCO3Molarity (mmol/L) = 399.6527017826 (charge/L) / |-1 (charge/mmol)|
= 399.6527017826 (charge/L) / 1 (charge/mmol)
= 399.6527017826 (mmol/L) - (step 3 reverse): HCO3 ion molarity > HCO3 ion mass concentration
Here we are converting molarity to mass concentration (because what we measure on the scale is mass, not molecules)
HCO3MassConcentration (mg HCO3/L) = HCO3Molarity (mmol/L) * 61.0174 (mg HCO3/mmol)
= 399.6527017826 (mmol/L) * 61.0174 (mg HCO3/mmol)
= 24385.7687657496 (mg HCO3/L) -
(step 2 reverse): HCO3 ion mass concentration > NaHCO3 salt mass concentration
Here we are converting salt hardness ion mass concentration to total salt mass concentration (because we aren’t adding/measuring individual ions, we are adding/measuring salt)
NaHCO3MassConcentration (mg NaHCO3/L) = HCO3MassConcentration (mg HCO3/L) / HCO3MassFraction (mg HCO3 / mg NaHCO3)with
HCO3MassFraction (mg HCO3 / mg NaHCO3) = (1 * 61.0174 (mg HCO3/mmol)) / 84.0066 (mg NaHCO3 / mmol)
= 0.7263405494 (mg HCO3 / mg NaHCO3)so
NaHCO3MassConcentration (mg NaHCO3/L) = 24385.7687657496 (mg HCO3/L) / 0.7263405494 (mg HCO3 / mg NaHCO3)
= 33573.4646590965 (mg NaHCO3/L) -
(step 1 reverse): NaHCO3 salt mass concentration > NaHCO3 salt mass
Here we are converting salt mass concentration to salt mass
(because we are making a specific amount of concentrate solution that requires a specific amount of salt)
NaHCO3Mass (mg NaHCO3) = NaHCO3MassConcentration (mg NaHCO3/L) * solution value (L)Now here is where you do some guess & check.
What we want is asolution volumethat we can easily measure on an espresso scale,
and anNaHCO3Massthat we can easily measure on a milligram scale.
“easy to measure” needs a definition… let’s make it mean that given our scales, we can measure the required quantities with an error less than +/-1%.Let’s assume the espresso scale has an accuracy of +/-0.04g or smaller and precision of 0.1g meaning we can meaningfully measure down to 0.1g. This means to make the
solution volumeeasy to measure, we need to measure at least 10g (.010 kg or L) (assuming water density of 1g/mL). Additionally we don’t want to make too much at once, so let’s limit thesolution volumeto about 500g (.500 kg or L) or so.Let’s also assume the milligram scale has an accuracy of +/-0.010g (10mg) and a precision of 0.001g (1mg)… so we can meaningfully measure around 0.010g (10mg) This means to make the
NaHCO3Masseasy to measure, we need to measure at least 1.000g (1000mg). Additionally, let’s assume our milligram scale has a maximum limit of 30g (30,000mg)I can tell you from experience that the water is going to be easier to measure accurately than the salts… so I recommend picking random water values and seeing how much salt they require. Pick the water value whose corresponding salt value is easiest to measure (I like numbers close to whole numbers)
so, for example, through trial and error I found 178.7g of water gives:
NaHCO3Mass (mg NaHCO3) = 33573.4646590965 (mg NaHCO3/L) * .1787 (L)
= 5999.5781345805 (mg NaHCO3)
= 5.9995781345805 (g NaHCO3)
Other Notes and Thoughts
- If purchasing from coffee roasters that are not local, then the water that they use may not match yours. The tasting notes may be different. Using this water would then try to match the water that your coffee roasters use to get the experience that the coffee roasters got.
- Rao/Perger water is suggested, but the formula is not introduced here because it may cause some detrimental effect for the boiler. If it is a cheap bonavita gooseneck kettle, it may be ok. But not others. Please check Gagne article or AWasteOfCoffee for the recipe.
- From strivefortone: The recommendation of 2:1 H:A ratio. 2:1 ratio comes from SCA specs and was explored a lot by Chris and Maxwell in ‘Water for Coffee’. Truth be told, you can go a lot higher in hardness (3:1, even 4:1 for some coffees) but this is mainly relevant for filter coffee where it is primarily consumed at lower concentrations. You may find that exploring the inverse of this with notably higher alkalinity works quite well in the context of espresso, especially with coffees with more acidic potential where the extra buffering capability can bring a pleasant balance. See the Direct Dosing table above. Fam’s 29th Wave is an example of high buffer water designed for light roast espresso. 15-20:60-70 are also common espresso water ratios.
- Direct Dosing vs Concentrates / Precision vs Accuracy - Do whatever works best for you. For myself, I use direct dosing. I tried using concentrates but I didn’t properly store the water. It seemed that there were some bacteria growing a month or two later. Storing the minerals dry seems to help a lot.
Additional Resources
- Jonathan Gagne Article - Water for Coffee Extraction
- Jim Schulman’s “Insanely Long Water FAQ”. It is very comprehensive and lives up to its name in length, but after reading it you will come out with a much better understanding of why you want to pay attention to the water you use for espresso.
- AWasteOfCoffee Methods on Mixing Water
Appendix:
Calculations for Determining how much to add to RO/DI Water for 100 GH and 50 KH.
Unit Balancing Convert KH to mol of HCO3 required and then convert that to KHCO3 in grams.
50 KH * 1 (mg/L CaCO3)/ 1 KH * 1 mg/L [HCO3] / 1.22 mg/L [CaCO3] * 1 mol [HCO3]/ 61.0168 g [HCO3] * 1 g / 1000 mg * 1 mol KHCO3 / 1 mol HCO3 * 100.115 g KHCO3 / 1 mol KHCO3
The result should be in g/L
The 1.22 is a magic number that derives from a Chemistry formula. The other values are found from the periodic table.
The following math calculations with are with commentary provided by strivefortone
If my target was 50 alkalinity, I’d assume that’s 50 mg/L as CaCO3.
First convert 50 mg/L as CaCO3 to mg/L as bicarbonate concentration
50 * 1.22 = 61 mg/L alkalinity as bicarbonate concentration.
61 is our target concentration
—
Let’s do potassium bicarbonate - KHCO3
Molar mass = 100.15 g/mol
That breaks down to atomic mass of each element:
Potassium (K) 39.0983
Hydrogen (H) 1.00794
Carbon (C) 12.0107
Oxygen (O) 15.9994 (multiply by 3)
We don’t care about the potassium for now, we want bicarbonate elements, so it’s 1.00794 + 1.00794 + (15.9994*3) for HCO3 = 61.01684
61.01684 is our atomic weight
Divide target concentration by HCO3 atomic weight
61 / 61.01684 = 0.99972401
0.99972401
—
Now you need to convert this to g/L so divide by 1000
0.99972401 / 1000 = 0.00099972
0.00099972
—
Now you need to multiply this by the molar mass (since K is still physically in the solution) to get grams needed per litre of DI water to achieve target mg/L of 61
0.00099972 * 100.115 = 0.100087369322961
0.100087369322961
—
Now multiply this by 10 to get grams needed for 10L batch of water with 50 alkalinity as CaCO3 when using KHCO3.
0.100087369322961 * 10 = 1
1g
—
Multiply this by 0.378 for 1 gallon …
1 * 0.378 = 0.38g for 1 gallon
So for completions sake - for a recipe with a KH:GH ratio of 1:2, with KHCO3 and MgSO4 - you have the calculation above for alkalinity, here’s how I would calculate hardness:
A 1:2 ratio would require 98.4ppm Mg as CaCO3
Convert to mg/L as the ion
98.4 / 4.22 = 24 mg/L Mg as the ion
(Note you can do this either way - convert as the ion to CaCO3 multiply by 4.22, convert CaCO3 to the ion divide by 4.22 - I’m following the convention for the first alkalinity calculation)
24 mg/L
—
So, MgSO4*7H20 (note: we’re using the heptahydrate because as soon as this sucker gets exposed to air it’s taking in all that oxygen — this is Epsom salts)
Molar Mass = 246.4746 g/mol
Magnesium (Mg) 24.3050
Hydrogen (H) 1.00794
Oxygen (O) (15.9994 multiply by 7)
Sulphur (S) 32.065
We’re only after magnesium for now, so:
Atomic weight = 24.3050
Divide target concentration by Mg atomic weight
24 / 24.3050 = 0.987451141740383
0.987451141740383
—
Now you need to convert this to g/L so divide by 1000
0.987451141740383 / 1000 = 0.000987451141740383
0.000987451141740383
—
Now you need to multiply this by the molar mass (since oxygen, hydrogen, and sulphur are still physically in the solution) to get grams needed per litre of DI water to achieve a target mg/L of 24:
0.000987451141740383 * 246.4746 = 0.243381625180004
0.243381625180004
—
Now multiply this by 10 to get grams needed for 10L batch of water with 98.4ppm Mg as CaCO3 when using MgSO4*7H20.
0.243381625180004 * 10 = 2.43g
2.43g
—
Convert to 1 gallon by multiplying by 0.378
2.43 * 0.378 = 0.92g
0.92g .92g